Science Modules / Oxygen
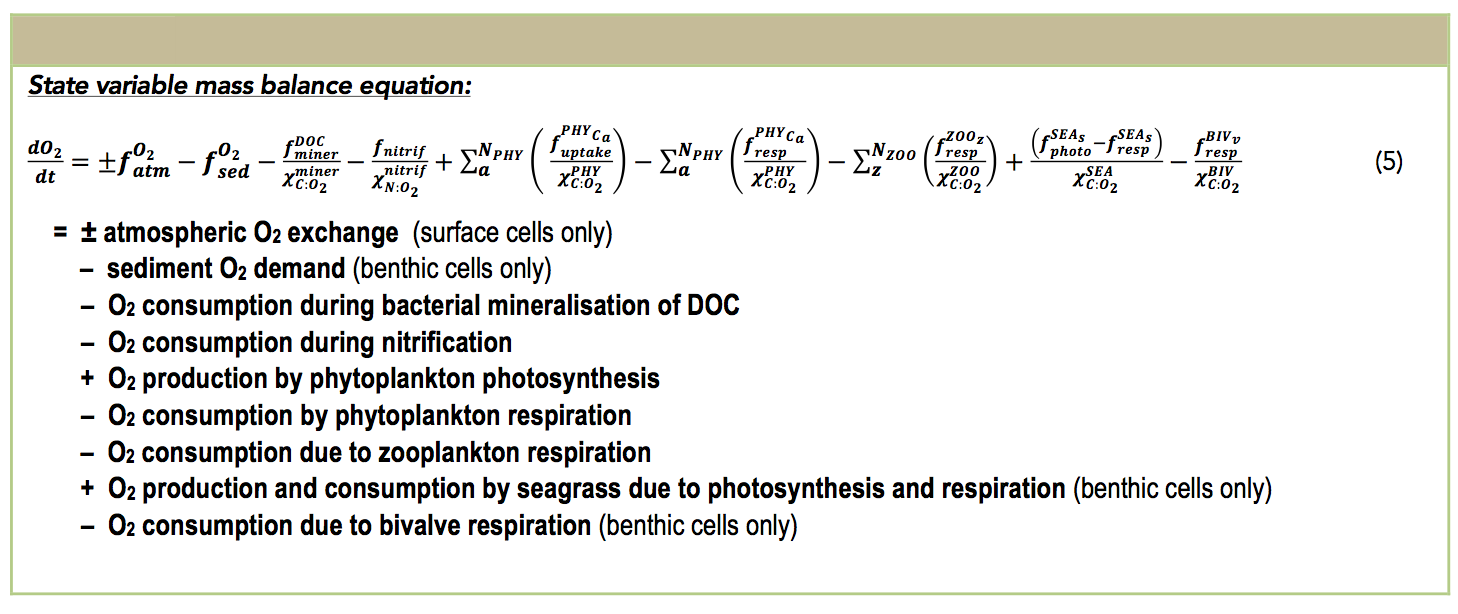
Dissolved Oxygen (DO) dynamics respond to processes of atmospheric exchange, sediment oxygen demand, microbial use during organic matter mineralisation and nitrification, photosynthetic oxygen production and respiratory oxygen consumption, chemical oxygen demand, and respiration by other biotic components such as seagrass and bivalves (see table below).
Other processes impacting the oxygen concentration include the breakdown of DOC by aerobic heterotrophic bacteria to CO2, whereby a stoichiometrically equivalent amount of oxygen is removed. Chemical oxidation, for example processes such as nitrification or sulfide oxidation, also consume oxygen dependent on the relevant stoichiometric factor. Photosynthetic oxygen production and respiratory oxygen consumption by pelagic phytoplankton is also included and is summed over the number of simulated phytoplankton groups. Seagrass interaction with the oxygen cycle is configurable within the model.
aed2_oxygen : oxygen solubility & atmospheric exchange
Atmospheric exchange is typically modelled based on Wanninkhof (1992) and the flux equation of Riley and Skirrow (1974) for the open water, and on Ho et al. (2011) for estuarine environments.
\begin{equation} F_{O_2} = k_{O_2} \left(C_{air} - C_{water}\right) \end{equation}where \(k_{O_2} (ms^{-1})\) is the oxygen transfer coefficient, \(C_{water} (gm^{-3})\) is the oxygen concentration in the surface waters near the interface and \(C_{air} (gm^{-3})\) is the concentration of oxygen in the air phase near the interface. A positive flux represents input of oxygen from the atmosphere to the water. \(C_{air}\) is dependent on temperature, T, salinity, S and atmospheric pressure, p, as given by \cite{Rile74}:
\begin{eqnarray} C_{air}\left(T,S,p \right) &=& 1.42763\: f\left(p\right) \exp \left\{ -173.4292+249.6339 \left[\frac{100}{\theta_k}\right] + 143.3483 \ln \left[ \frac{\theta_k}{100}\right] \right. \nonumber \\ &-& \left. 21.8492 \left[\frac{\theta_k}{100}\right] + S\left(-0.033096 +0.014259 \left[\frac{\theta_k}{100}\right] -0.0017 \left[\frac{\theta_k}{100}\right]^2 \right) \right\} \end{eqnarray} where \(\theta_k\) is temperature in degrees Kelvin, salinity is expressed as parts per thousand and atmospheric pressure is in kPa. The pressure correction function, f(p) varies between one and zero for the surface and high altitudes respectively: \begin{equation} f\left(p\right)=\frac{p_H}{p_{SL}}\left[1-\frac{p_{vap}}{p_H}\right]/\left[1-\frac{p_{vap}}{p_{SL}}\right]. \end{equation}aed2_oxygen : sediment oxygen demand
Modelling sediment oxygen demand can take a variety of forms. The simplest form in AED2 is the SOD varies as a function of the overlying water temperature and dissolved oxygen levels.
\begin{equation} f_{O_2}^{DSF}(T,DO)= S_{SOD}\:f_{SED}^{T2}(T)\:f_{SOD}^{DO1}(DO) \end{equation} where \(S_{SOD}\) is a fixed oxygen flux across the sediment-water interface and \(K_{DO_{SOD}}\) is a half-saturation constant for the sediment oxygen demand.
other oxygen sources/sinks (optional)
Variable Summary & Setup Options
| Variable Name | Description | Units | Variable Type | Core/Optional |
|---|---|---|---|---|
OXY_oxy |
dissolved oxygen concentration | $$mmol\,m^{-3}$$ | pelagic | core |
| Variable Name | Description | Units | Variable Type | Core/Optional |
|---|---|---|---|---|
OXY_oxysat |
dissolved oxygen saturation | % | pelagic diagnostic | core |
OXY_atm_oxy_flux |
O2 exchange across atm/water interface | $$mmol\,m^{-2}\,day^{-1}$$ | - | - |
| Parameter Name | Description | Units | Parameter Type | Default | Typical Range | Comment |
|---|---|---|---|---|---|---|
oxy_initial |
initial dissolved oxygen (DO) concentration | $$mmol\,m^{-3}$$ | float | 0-400 | Note: will be overwritten by GLM or TFV IC | |
Fsed_oxy |
sediment oxygen demand (SOD) | $$mmol\,m^{-2}\,day^{-1}$$ | float | -100 | Note: unused if Fsed_oxy_variable is activated via aed2_sedflux | |
Ksed_oxy |
half-saturation concentration of oxygen sediment flux | $$mmol\,m^{-3}$$ | float | 50 | 10-100 | Changes the sensitivity of the oxygen flux to the overlying oxygen concentration |
theta_sed_oxy |
Arrhenius temperature multiplier for sediment oxygen flux | - | float | 1e+00 | 1.04 - 1.12 | Changes the sensitivity of the oxygen flux to the overlying temperature |
Fsed_oxy_variable |
oxygen sediment flux variable link | - | string | ' | e.g.: SDF_Fsed_oxy | will use the value supplied by the aed2_sedflux model for Fsed_oxy; use this option to allow for spatial or temperal variation |
oxy_min |
minimum dissolved oxygen (DO) concentration | $$mmol\,m^{-3}$$ | float | Optional variable to enforce negative number clipping | ||
oxy_max |
maxmium dissolved oxygen (DO) concentration | $$mmol\,m^{-3}$$ | float | - | 1000 | Optional variable to enforce high number clipping |
An example nml block for the oxygen module is shown below:
&aed2_oxygen
oxy_initial = 225.0
Fsed_oxy = -40.0
Ksed_oxy = 100.0
theta_sed_oxy = 1.08
! Fsed_oxy_variable = 'SDF_Fsed_oxy'
oxy_min = 0
oxy_max = 500
/
Examples
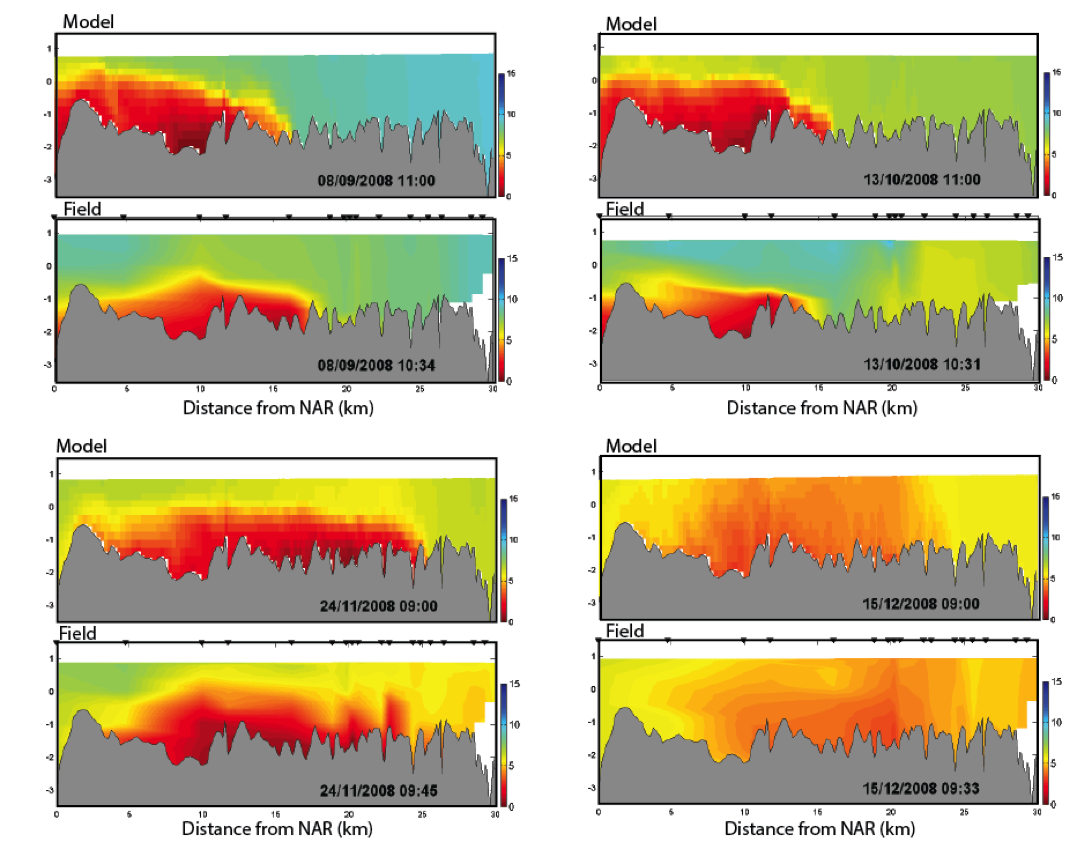
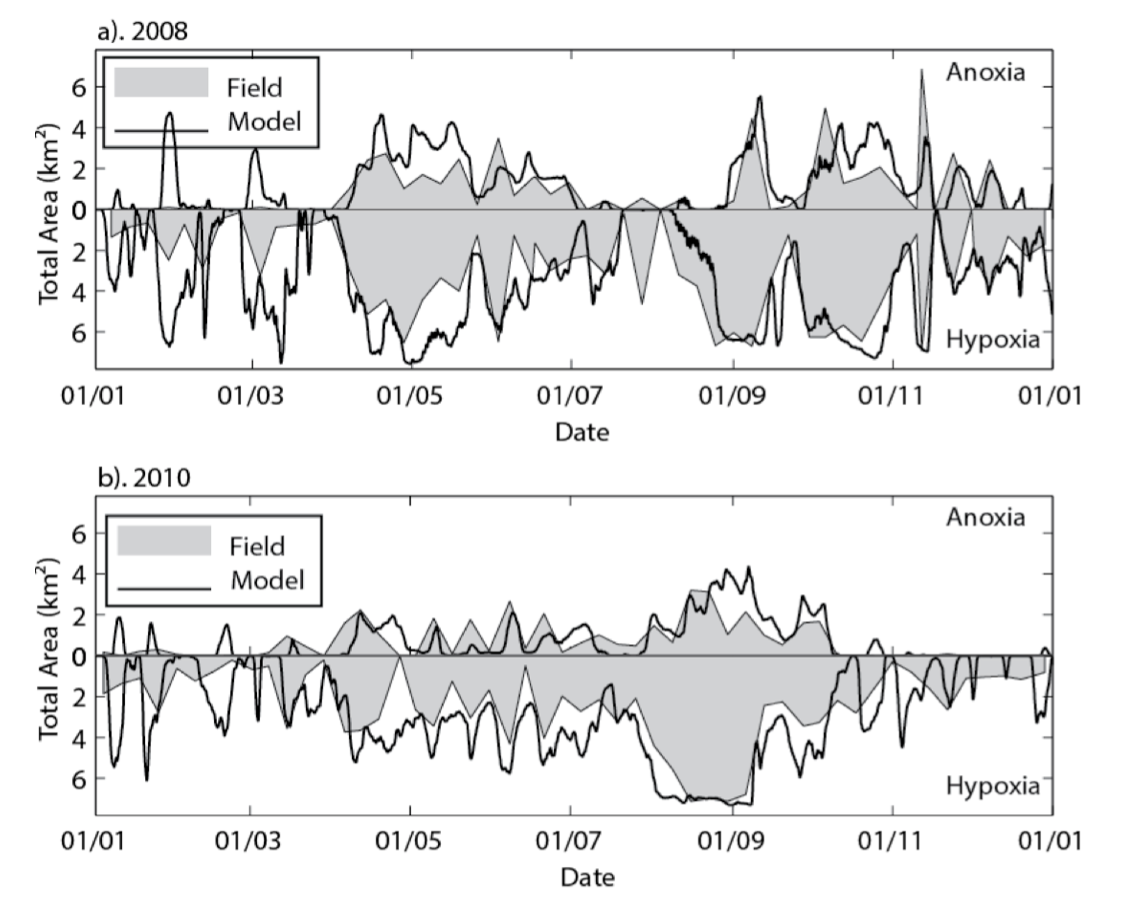
Example 1: Cross-section plots comparing modelled and field salinity (psu, left) and oxygen (mg/L, right) for Sep-Dec 2008 at Swan River. The field plots are based on a contouring around 20 profile data locations.
Publications & References
Wanninkhof, R.. 1992. Relationship between windspeed and gas exchange over the ocean. J. Geophys. Res. (Oceans) 97(C5): 7373– 7382.
Riley, J.P. & Skirrow, G.. 1974. Chemical Oceanography Academic Press, London.
Ho, D.T., Schlosser P., & Orton, P.M., 2011. On factors controlling air-water gas exchange in a large tidal river. Estuaries and Coasts. 34:1103-1116.